 The UM research team develops a novel aqueous two-phase system for large-scale cultivation and rapid activation of therapeutic cells
The UM research team develops a novel aqueous two-phase system for large-scale cultivation and rapid activation of therapeutic cells
A research team led by Wang Chunming, professor in the Institute of Chinese Medical Sciences and the State Key Laboratory of Mechanism and Quality of Chinese Medicine at the University of Macau (UM), has developed a novel engineered cell culture system based on the physicochemical principle of phase separation, which can significantly shorten the time required for activating immune cells. The research has recently been published in Cell Biomaterials, a sibling journal to the prestigious international journal Cell, and has prompted a translational collaboration with a clinical research centre in the Guangdong-Hong Kong-Macao Greater Bay Area.
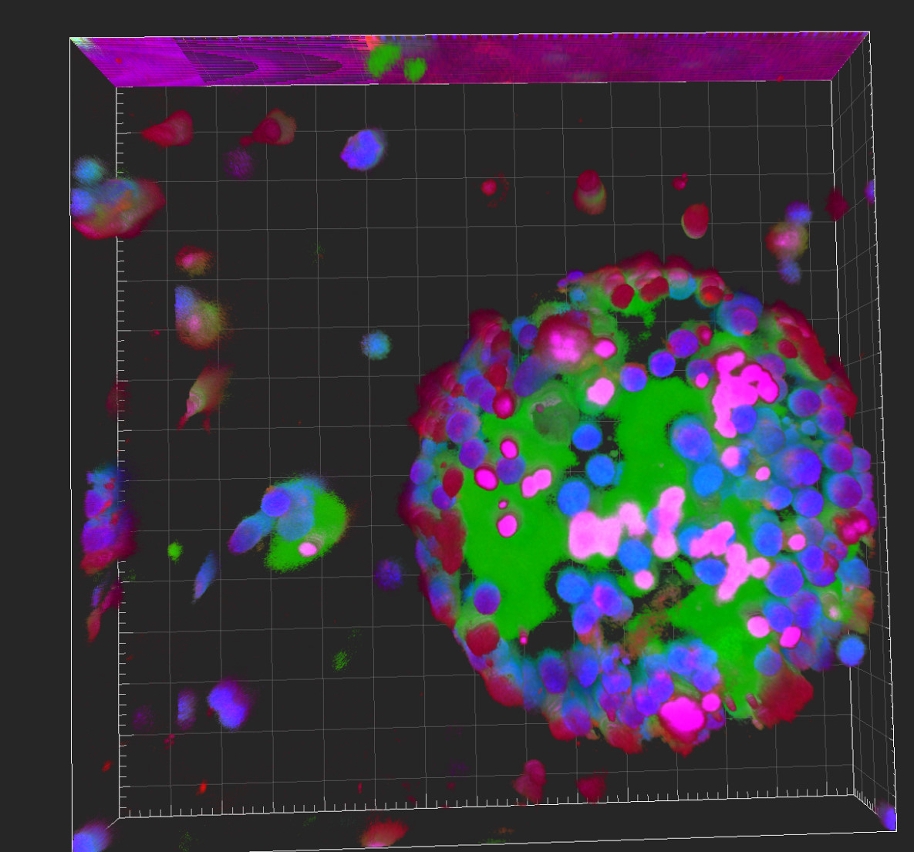
Cell therapy offers new possibilities for treating various diseases and repairing damaged tissue. However, the lengthy activation process of patient-derived cells remains a critical bottleneck in clinical translation. Inspired by the fundamental physical principle that biomolecules may form condensates intracellularly to accelerate biochemical reactions, the UM research team attempted to create a similar system on the cell surface to accelerate activation. They used two biocompatible polymers—polyethylene glycol (PEG) and dextran (DEX)—to construct an aqueous two-phase system (ATPS). By selectively co-partitioning cells and activating factors, the system enriched activating factors in the cell-partitioned phase at concentrations that exceeded conventional levels, thereby rapidly inducing cells to the desired phenotypes. The research team tested the system on two representative model cell types, macrophages and T cells, and found that it could reduce activation time by up to 80% while increasing target gene expression levels by tenfold. Interestingly, this engineered system, which encapsulates cells in a phase-separation environment, markedly enhanced liquid-liquid phase separation of key proteins (such as IKBKG) in relevant signalling pathways of immune activation, with the entire activation process based on clear and controllable regulatory mechanisms. As the system comprises only two inexpensive, safe, and long-approved biomaterials that are widely endorsed by regulatory agencies in multiple countries, it is expected to facilitate regulatory approval applications and elevate interest from clinical and industrial collaborators for the application.
The co-first authors of the study are Li Yuwei, a master’s graduate from the UM Institute of Chinese Medical Sciences (ICMS), and Wang Anheng, a postdoctoral researcher in ICMS. Associate Professor Massimiliano Galluzzi from Shenzhen University, Dr Jordi Esquena from the Institute of Advanced Chemistry of Catalonia in Spain, and Professor Dong Lei from the School of Life Sciences at Nanjing University, among others, also made substantial contributions to the study. The research was funded by the Science and Technology Development Fund of the Macao SAR (File Nos.: 0001/2021/AKP, 0024/2023/AFJ, 005/2023/SKL) and the University of Macau (File Nos.: MYRG-GRG2024-00189-ICMS-UMDF, MYRG-GRG2023-00136-ICMS-UMDF, MYRG-CRG2023-00009-IAPME). The full version of the research article can be accessed at: https://www.cell.com/cell-biomaterials/fulltext/S3050-5623(25)00168-0.